A study recently published in the journal
PLoS Pathogens in
April of 2017 provided startling evidence demonstrating for the first
time that the commonly-prescribed anti-diabetic drug metformin exerted
significant antiviral effects in dengue virus-infected human liver cells
that was dependent on activation of the master metabolic regulator AMPK
[59]. The authors showed that an in increase HMG-CoA reductase (HMGCR)
activity, a target of AMPK, was associated with dengue virus
(DENV)-infected cells, AMPK activation was reduced in DENV-infected
cells at 12 and 24 hours post infection (hpi), and metformin
significantly decreased the number of infected cells, viral yield, and
viral genome copies, leading the authors to conclude that
metformin-induced AMPK activation generates a strong antiviral effect
against DENV [59]. Interestingly, as discussed below, recent efforts
funded by the U.S. and British governments, the Bill & Melinda Gates
Foundation, and the Google health spin-off Verily have sought to
decrease the spread of dengue and Zika viruses through the coordinated
release of female and/or male mosquitoes (called
Aedes aegypti)
that were purposely infected with a bacterium that inhibits the
mosquito’s ability to transmit the two viruses to humans [1]. Studies
have shown that this bacterium, called
Wolbachia, enhances the
mosquito’s immune response by increasing the levels of reactive oxygen
species (ROS), thus enhancing inhibition of dengue virus replication
[30]. Because AMPK is activated by cellular stress (e.g. ROS increase,
intracellular calcium [Ca2+] increase, AMP/ATP ratio increase, etc.),
has been found in
Aedes aegypti (
Ae. aegypti), and
AMPK activation by stress-inducing compounds (e.g. resveratrol)
increased average life span and enhanced the immune response in
Ae. aegypti
in an AMPK-dependent manner, the recent finding that metformin also
inhibits DENV replication in human cells in an AMPK-dependent manner
provides compelling evidence that the anti-viral effects of AMPK
activation likely crosses species boundaries [31]. Additionally,
metformin has recently been shown to beneficially alter gene splicing,
activate AMPK, and ameliorate accelerated aging defects in cells derived
from Hutchison-Gilford progeria syndrome patients (HGPS), as I first
hypothesized and published in 2014 [60-62]. AMPK activation also
promotes oocyte meiotic induction and maturation (in preparation for
oocyte activation) and AMPK has recently been found localized across the
entire acrosome in human spermatozoa [53,55]. The induction of cellular
stress (e.g. ROS, intracellular Ca2+, and/or AMP/ATP ratio increase)
also promotes oocyte meiotic induction/maturation, oocyte activation,
and the acrosome reaction in human sperm, processes critical for the
creation of all human life [53,56,63]. As further discussed below, such
interconnectedness implicates AMPK as a central mediator in the
promotion of lifespan and healthspan, amelioration of pathological
aging, the mounting of an effective immune response, and the creation of
all human life.
Transmission of DENV by mosquito vectors including
Ae. aegypti
may lead to febrile illness (known as dengue fever) in humans
characterized by fluid retention, respiratory distress, and/or organ
impairment [59]. There are four DENV serotypes (DENV 1-4) and several
structural (capsid (C), membrane (M), and envelope (E)) and
nonstructural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5) are
encoded by the DENV genome. Interestingly, lipids and the formation of
replication complexes (i.e. membranous compartments) have been shown to
play important roles in the DENV replication cycle whereas the viral
protein NS4A is directly associated with DENV-associated membrane
rearrangement [59]. The authors first showed that AMPK activation was
reduced in DENV-infected Huh7 cells (serotype 2 and 4, 2/4) at 12 and 24
hpi compared to mock-treated cells. Importantly, metformin enhanced
AMPK activation in DENV 2/4-infected cells compared to
mock/vehicle-treated cells, reduced NS3 levels compared to
vehicle-treated cells, and reduced the levels of the viral structural
proteins E and prM. DENV 2/4 infection also increased the activity of
HMGCR (a rate-controlling enzyme of the cholesterol biosynthetic
pathway) in vehicle-treated cells compared to mock-infected cells,
whereas metformin disturbed the co-localization between HMGCR and NS4A
or NS3, disrupted replicative complex integrity, and decreased the
levels of the viral proteins NS4A and NS3. Furthermore, metformin also
led to a reduction in the levels of DENV dsRNA, indicating that
metformin exerted a strong antiviral effect against DENV [59].
Indeed, 24 hour treatment of DENV2/4-infected Huh7 cells with
metformin led to a reduction in the amount of infected cells, decreased
the viral yield up to one logarithm, and reduced NS1 secretion up to
90%. Metformin also led to a dose-dependent reduction in viral genome
copies (up to 0.7 logarithm for DENV2 and 1.5 logarithm for DENV4)
compared to non-treated cells [59]. Additionally, treatment of
DENV-infected cells with the AMPK inhibitor compound C (CC) increased
viral infection compared to non-treated cells and CC-induced AMPK
inhibition increased viral genome copies up to a half logarithm in DENV
2- and up to 0.7 logarithm in DENV 4-infected cells, indicating that
metformin’s potent antiviral effects against DENV infection and
replication is dependent on AMPK activation [59].
Metformin’s AMPK-dependent antiviral effects against DENV in human
liver cells likely represent a common mechanism that crosses species
boundaries to effectuate viral eradication. As noted above, current
efforts by organizations including the Bill and Melinda Gates foundation
and the Google health spin-off Verily has focused on reducing
transmission of dengue and Zika viruses by infecting the mosquito
Ae. aegypti with the bacterium
Wolbachia,
leading to inhibition of viral replication. As explained below,
inhibition of DENV replication in both mosquitoes and in human cells is
likely orchestrated by stress-induced AMPK activation.
Although the method through which
Wolbachia is transmitted to mosquito offspring has been well studied, how
Wolbachia
infection leads to a decrease in dengue and Zika virus replication in,
and transmission by, mosquitoes is heavily debated. Interestingly,
although
Wolbachia does not naturally occur in
Ae. aegypti,
Wolbachia
appears to impart beneficial immunomodulatory effects in these
mosquitoes not unlike that of certain beneficial bacteria that normally
colonize the human gut. Such bacteria, also known as “probiotics”, are
typified by the genera
Lactobacillus and Bifidobacterium and
have been shown to significantly enhance natural and acquired immunity,
induce reactive oxygen species (ROS) generation and the upregulation of
Nrf2-dependent cytoprotective genes, produce short chain fatty acids
(SCFAs) that promote T cell differentiation, and produce antimicrobial
peptides that exhibit bactericidal activity [4-7]. Interestingly, the
induction of cellular stress via ROS generation has been shown to
activate the master metabolic regulator AMPK and AMPK activation has
been shown to be critical for T cell activation and the mounting of an
effective immune response
in vivo to viral and bacterial
infections [8-10]. Indeed, acetate, propionate, and butyrate, three
SCFAs abundantly produced by probiotic bacteria, each activate AMPK,
indicating that the immunomodulatory effects of probiotic bacteria are
dependant on cellular stress-induced activation of AMPK [11].
Furthermore, AMPK has recently been discovered in
Ae. aegypti and activation of AMPK in
Ae. aegypti by compounds that induce cellular stress leads to an enhanced immune response and increased longevity in
Ae. aegypti (see below), implicating the provocative assertion that inhibition of dengue and Zika virus replication via
Wolbachia infection occurs as a result of cellular stress-induced activation of AMPK [12].
Wolbachia, which are found in reproductive tissues of arthropods, often engage in a mutualistic relationship with its host. Indeed,
Wolbachia has been shown to induce resistance to RNA viral infections in the model organism
Drosophila melanogaster [13].
D. melanogaster (also known as the fruit fly)
has
been studied extensively in biological research and activation of AMPK
has been shown to extend lifespan as well as slow aging in
D. melanogaster
through autophagy induction via upregulation of Atg1 (ULK1 in mammals),
reduction of insulin-like peptide levels in the brain, and an increase
in 4E-BP [14,15].
Interestingly, insulin-like peptides and hyperphosphorylation of
4E-BP (i.e. inactivation) is positively associated with target of
rapamycin (TOR) activation and egg development in
Ae. aegypti [16].
Mammalian
target of rapamycin (mTORC1/TORC1) is a protein kinase that plays a
critical role in promoting mRNA translation and is the primary target of
rapamycin, a macrolide drug that extends lifespan in several model
organisms including normal genetically heterogeneous mice and
D. melanogaster [17,18]. Rapamycin was shown to delay yolk deposition in control
Ae. aegypti eggs
and inhibit insulin-mediated 4E-BP (a negative regulator of
translation) phosphorylation. Silencing of 4E-BP also led to a reduction
in lifespan of adult female mosquitoes, mirroring results obtained via
AMPK activation in
D. melanogaster [15,16,19]. Strikingly, in addition to delaying egg maturation in
Ae. aegypti, rapamycin has also been shown to dramatically increase the number of
Wolbachia in
D. melanogaster oocytes via TORC1 inhibition, whereas somatic hyperactivation of TORC1 decreases
Wolbachia titer in oocytes [20]. Interestingly, ablation of insulin-producing cells in the
D. melanogaster brain abolished the yeast-induced reduction of
Wolbachia in oocytes, mirroring previous results showing that AMPK-induced lifespan extension in
D. melanogaster is associated with a decrease in insulin-like peptides in the brain [15,20].
Because rapamycin, similar to AMPK activation by diverse compounds,
induces autophagy, inhibits mTOR/TORC1, promotes mitochondrial
biogenesis, and extends lifespan in several model organisms, it would
expected that rapamycin would also induce activation of AMPK, likely via
the induction of cellular stress (i.e. increase in the levels of ROS,
intracellular Ca2+ increase, increase in the AMP:ADP/ATP ratio,
etc). Indeed, two recent publications clearly demonstrated that
rapamycin potently induced the activation of AMPK
in vivo in
normal elderly mice, along with an upregulation of ULK1 (mammalian
orthologue of Atg1 critical for the induction of autophagy) and PGC-1a
(a transcription factor essential for promoting mitochondrial
biogenesis) [21,22]. As rapamycin has been shown to increase the number
of
Wolbachia in
D. melanogaster oocytes, delay egg maturation and inhibit insulin-mediated 4E-BP phosphorylation in
Ae. aegypti, and increase lifespan in
D. melanogaster, the recent finding that activation of AMPK in
Ae. aegypti
also increases lifespan and improves the immune response (see below)
further bolsters the notion that activation of AMPK via the induction of
cellular stress likely promotes lifespan extension, immune system
enhancement, and
Wolbachia propagation in oocytes in both
D. melanogaster and
Ae. aegypti. Moreover, because certain bacteria (e.g.
Lactobacillus)
that colonize the human gut induces beneficial immune responses by
inducing cellular stress (e.g. increased ROS levels) and also produce
compounds that activate AMPK, the inhibition of dengue and Zika virus
replication in, and transmission by,
Ae. aegypti is likely the result of a
Wolbachia-induced cellular stress response, leading to the up regulation of anti-viral mechanisms that are likely modulated by AMPK.
Indeed, a recent study by Wong et al. demonstrated that ROS/oxidative stress is positively correlated with
Wolbachia-mediated antiviral protection in
D. melanogaster
[23]. The authors of the study observed that H2O2 (hydrogen peroxide)
was increased 1.25- to 2- fold in flies that harbored protective strains
of
Wolbachia (e.g.
wMelCS,
wRi,
wAu) compared to
Wolbachia-free
controls. Interestingly, flies with a null mutation in Cu/Zn SOD (an
antioxidant enzyme) exhibited elevated endogenous levels of oxidative
stress that mimicked
Wolbachia-induced oxidative stress. In these
Wolbachia-free Cu/Zn SOD mutant flies, a 70% survival rate was observed at 4 days post-infection after
Drosophila C
virus infection compared to a less than 10% survival rate for
non-mutant flies, indicating that elevated levels of oxidative stress
induced by protective
Wolbachia strains confers a survival
advantage by decreasing susceptibility to viral infection via activation
of signaling pathways that potentiate anti-viral immune responses [23].
As noted above, ROS/oxidative stress has been shown to activate AMPK and AMPK activation in both
D. melanogaster and
Ae. Aegypti leads
to an increase in lifespan [8,12,15]. Interestingly, Sykiotis et al.
showed that oxidants/oxidative stress promote lifespan extension in
D. melanogaster
males by activating the Nrf2 (CncC) pathway, a master antioxidant
transcription factor that induces the expression of several antioxidant
genes including NAD(P)H quinone oxidoreductase 1 (NQO1) and heme
oxygenase-1 (HO-1) [24]. Curiously, Sykiotis et al. also observed that
the synthetic dithiolthione oltipraz, which has been shown to activate
AMPK, inhibit HIV-1 replication, and reverse accelerating aging defects
in Hutchinson-Gilford progeria syndrome (see below), also induced Nrf2
signaling in flies [24-27]. AMPK has also been shown to increase the
transcriptional activity of Nrf2 and increase Nrf2 nuclear retention via
phosphorylation, suggesting that AMPK may represent a central node in
ROS/oxidative stress-induced immune and anti-viral responses [28,29].
A recent study by Pan et al. showed that infection of
Ae. Aegypti with
Wolbachia (
wAlbB
strain) led to a ROS/oxidative stress-induced upregulation of genes
associated with immunity and reduction-oxidation, thus enhancing
inhibition of dengue virus replication [30]. Several gene transcripts
related to the immunity and redox/stress/mitochondrion group were
upregulated in
Wolbachia-infected
Ae. aegypti females before blood feeding and after infection with DENV serotype 2, including the antimicrobial peptides cecropin D (
CECD) and defensin C (
DEFC) as
well as the antioxidants glutathione peroxidase, CuZnSOD, MnSOD, and
glutathione peroxidase [30]. A significant increase in the levels of
H2O2 and in the transcript abundance of both NADPH oxidase M (NOXM) and
dual oxidase 2 (DUOX2), two enzymes that generate ROS, were also
observed in
Wolbachia-infected mosquitoes compared to control
mosquitoes. Interestingly, the expression of several Toll pathway (a
pathway that mediates the production of antioxidants and antimicrobial
peptides) marker genes as well as the antimicrobial peptides
CECD and
DEFC
were increased by the addition of H2O2 in a sugar solution given to
female control/uninfected mosquitoes [30]. This effect was also mirrored
in
Wolbachia-infected female mosquitoes, wherein silencing of
NOXM and DUOX2 deactivated the Toll pathway and suppressed the
expression of CECD and DEFC, indicating that
Wolbachia-induced
ROS is required for activation of the Toll pathway and induction of
antimicrobial peptides. Importantly, individual or double RNAi-induced
knockdown of DEFC and CECD significantly increased viral titers of DENV
serotype 2 in
Wolbachia-infected mosquitoes compared to controls, providing further evidence that
Wolbachia-induced
ROS leads to a beneficial cellular response characterized by
antimicrobial-mediated inhibition of viral replication [30].
Because
Wolbachia-induced ROS/oxidative stress leads to beneficial anti-viral cellular responses in both
D. melanogaster and
Ae. aegypti and because AMPK activation, which increases Nrf2 transcriptional activity, leads to increased lifespan in
D. melanogaster, it would be expected that cellular stress-induced AMPK activation in
Ae. aegypti
would also lead to an increase in lifespan as well as an enhanced
immune response. Indeed, Nunes et al. showed that autophagy, immune
system activation, and the average lifespan of
Ae. Aegypti (a
vector for dengue as well as chikungunya and Zika virus) is increased by
feeding mosquitoes several plant-based polyphenols [31]. Interestingly,
administration of resveratrol (derived from grapes), quercetin (found
in many fruits and vegetables), epigallocathechin-3-gallate (EGCG, found
in green tea), and genistein (found in soybeans) each increased average
lifespan for male and female mosquitoes compared to controls. Insects
fed resveratrol also displayed higher levels of AMPK
phosphorylation/activation compared to controls and compound C, a
pharmacological inhibitor of AMPK, blocked the suppression of
triglyceride (TG) content induced by resveratrol [31]. Resveratrol
treatment also led to immunomodulatory effects, with reduced bacterial
populations for female mosquitoes compared to controls, an effect that
was mimicked by AICAR (a prototypical AMPK activator) but inhibited by
compound C. Interestingly, resveratrol only slightly decreased bacterial
populations
in vitro, indicating that the effects of
resveratrol are indirect and likely associated with activation of the
immune response in mosquitoes. Autophagy was also stimulated in
resveratrol- and AICAR-fed mosquitoes which was abolished by silencing
of AMPK [31].
Interestingly, each of the polyphenols used in that study
(resveratrol, quercetin, epigallocathechin-3-gallate, and genistein)
have each been shown to induce cellular stress (e.g. ROS generation),
similar to
Wolbachia-induced ROS generation, in a number of
mammalian cells in addition to activating AMPK [32-35]. As AMPK is
activated by a number of different compounds (polyphenols, bacterial
metabolites, metformin etc.) and methodologies (e.g. electrical
stimulation) that induce cellular stress, it is likely that
Wolbachia-induced inhibition of dengue and Zika virus replication in and transmission by
Ae. Aegypti
is also modulated by AMPK, a master metabolic regulator that increases
lifespan and improves immune and antioxidant responses in another
Wolbachia-infected insect,
D. melanogaster
[36,37]. Although Nunes et al. did not determine if the polyphenols
tested inhibited dengue virus replication in or transmission by
Ae. Aegypti,
recent studies have shown that in mammalian cells quercetin and analogs
of resveratrol exhibit significant inhibitory activities against dengue
virus type 2 [38,39]. EGCG has also recently been shown to inhibit Zika
virus entry in Vero E6 cells (albeit at higher concentrations),
indicating that cellular stress-induced activation of AMPK may represent
a common mechanism of action that spans species boundaries to prevent
the replication and/or transmission of dengue and Zika viruses
[40]. Additionally, salidroside (derived from the plant
Rhodiola rosea) and curcumin (derived from the plant
Curcuma longa) have
both been shown to exhibit anti-dengue virus activity in vitro and
induce AMPK activation in vivo, providing further evidence that many
structurally diverse compounds likely share a common mechanism of
stress-induced AMPK activation to effectuate antiviral responses
[41-44].
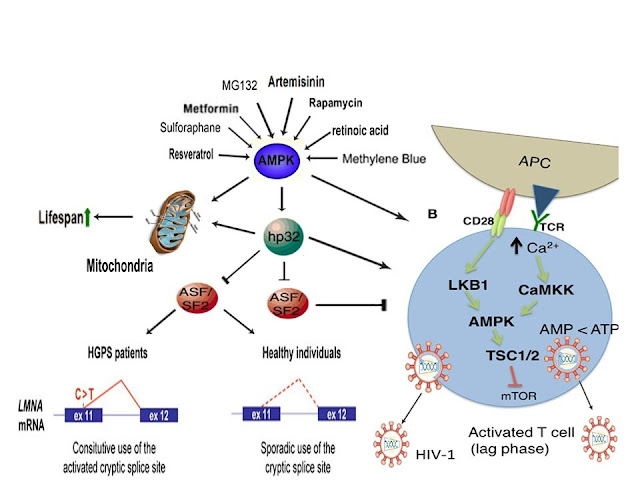
Remarkably, cellular stress-induced activation of AMPK (i.e. “Shock”) appears to link the antiviral effects mediated by a
Wolbachia-induced increase in ROS production in
A. aegypti with
the amelioration of accelerated cellular aging defects in the genetic
disorder Hutchinson-Gilford progeria syndrome (HGPS), the reactivation
of latent HIV-1 in infected T cells (to facilitate immune system
detection and virus destruction), oocyte meiotic resumption, and the
acrosome reaction in sperm (i.e. “Live”).
Indeed, the proteasome inhibitor MG132 has recently been shown to
inhibit progerin (the toxic protein that causes accelerated cellular
aging defects) in fibroblasts derived from HGPS patients, inhibit
dengue, West Nile, and Yellow fever viruses, and activate AMPK [45-47].
1α,25-dihydroxyvitamin D3, the most potent metabolite of vitamin D, has
also been shown to delay premature senescence and significantly improve
accelerated aging in patient-derived HGPS cells, reduce dengue virus
infection in human myelomonocyte and hepatic cell lines, and activate
AMPK [48-50]. Also, as discussed above, metformin has recently been
shown to beneficially alter gene splicing, activate AMPK, and ameliorate
accelerated aging defects in cells derived from Hutchison-Gilford
progeria syndrome patients (HGPS) and significantly inhibit dengue virus
infection and replication in human liver cells
[59,60-62]. Interestingly, nuclear aging defects in HGPS patient-derived
cells were also reversed via activation of the antioxidant
transcription factor Nrf2 by oltipraz. Oltipraz has also been shown to
activate AMPK, inhibit HIV-1 replication, and induce Nrf2 signaling in
D. melanogaster [24-27].
Furthermore, just as
Wolbachia-induced ROS/hydrogen peroxide
(H2O2) generation (i.e. “Shock”) leads to a compensatory upregulation
of antimicrobial peptides and an enhanced antiviral immune response in
Ae. Aegypti (i.e.
“Live”), the exposure of HIV-1 latently infected monocyte or lymphocyte
cell lines to H2O2 has also been shown to reactivate HIV-1 [51].
Additionally, ROS induces activation of AMPK and AMPK plays a critical
role in T cell activation (and thus reactivation of latent HIV-1 that
resides in T cell cells) [8,52,64].
AMPK activation has also been shown to play a critical role in the
initiation of oocyte meiotic resumption and maturation (in preparation
for oocyte activation) and the free radical-generating agent menadione
(i.e. “Shock”) has been shown to induce AMPK-dependent meiotic
resumption in cumulus-enclosed and denuded mouse oocytes (i.e. “Live”)
via promotion of oxidative stress [53]. Lastly, ROS also plays a
critical role in the induction of the acrosome reaction in sperm, a
process that facilitates oocyte penetration through release of
hydrolytic enzymes and is indispensable, along with oocyte activation,
for the creation of all human life outside of a clinical setting
[54]. Strikingly, AMPK has recently been found for the first time to be
localized across the entire acrosome in human sperm and both H2O2/ROS
and vitamin D (i.e. “Shock) have been shown to induce the acrosome
reaction in human sperm (i.e. “Live”), providing compelling evidence
that cellular stress-induced AMPK activation is critical for the
induction of the acrosome reaction in human sperm and the creation of
human life [55-57].
The antiviral effects mediated by
Wolbachia-induced ROS
generation paints a clear yet provocative portrait that cellular-stress
mediated activation of AMPK represents a common mechanism of action that
spans species boundaries, beneficially modulating cellular processes
that are involved in the immune response, fertilization, and aging
itself. Indeed, structurally distinct compounds including metformin,
MG132, and vitamin D have been shown to exert potent antiviral effects
against dengue virus and significantly improve accelerated aging defects
in HGPS. More tellingly, H2O2/ROS/oxidative stress has been shown to
reactivate latent HIV-1, promote oocyte meiotic resumption, and induce
the acrosome reaction in human sperm. Because AMPK is critical for
oocyte meiotic resumption (and hence oocyte activation) and AMPK has
recently been found for the first time to be localized across the entire
acrosome in human sperm, AMPK activation may indeed be critical for the
creation of all human life, as originally proposed in my recent
publication [58]. Perhaps beyond perplexing on first glance,
Wolbachia, mosquitoes, and the creation of all human life are likely connected.
https://www.linkedin.com/pulse/metformin-shown-first-time-inhibit-dengue-virus-human-finley
References
- http://www.reuters.com/article/us-health-dengue-mosquitoes-idUSKCN12Q1PE
- https://www.bloomberg.com/news/articles/2016-10-06/alphabet-s-verily-joins-zika-fight-with-sterile-mosquito-lab
- Werren JH. Biology of Wolbachia. Annu Rev Entomol. 1997;42:587-609.
- Castillo NA, Perdigón G, de Moreno de Leblanc A. Oral
administration of a probiotic Lactobacillus modulates cytokine
production and TLR expression improving the immune response against
Salmonella enterica serovar Typhimurium infection in mice. BMC
Microbiol. 2011 Aug 3;11:177.
- Gill HS, Rutherfurd KJ, Prasad J, Gopal PK. Enhancement of natural
and acquired immunity by Lactobacillus rhamnosus (HN001), Lactobacillus
acidophilus (HN017) and Bifidobacterium lactis (HN019). Br J Nutr. 2000
Feb;83(2):167-76.
- Jones RM, Desai C, Darby TM, et al. Lactobacilli Modulate
Epithelial Cytoprotection through the Nrf2 Pathway. Cell Rep. 2015 Aug
25;12(8):1217-25.
- Park J, Kim M, Kang SG, et al. Short-chain fatty acids induce both
effector and regulatory T cells by suppression of histone deacetylases
and regulation of the mTOR-S6K pathway. Mucosal Immunol. 2015
Jan;8(1):80-93.
- Mungai PT, Waypa GB, Jairaman A, et al. Hypoxia triggers AMPK
activation through reactive oxygen species-mediated activation of
calcium release-activated calcium channels. Mol Cell Biol. 2011
Sep;31(17):3531-45.
- Tamás P, Hawley SA, Clarke RG, et al. Regulation of the energy
sensor AMP-activated protein kinase by antigen receptor and Ca2+ in T
lymphocytes. J Exp Med 2006;203(7):1665–70.
- Blagih J, Coulombe F, Vincent EE, et al. The energy sensor AMPK
regulates T cell metabolic adaptation and effector responses in vivo.
Immunity. 2015 Jan 20;42(1):41-54.
- Elamin EE, Masclee AA, Dekker J, Pieters HJ, Jonkers DM.
Short-chain fatty acids activate AMP-activated protein kinase and
ameliorate ethanol-induced intestinal barrier dysfunction in Caco-2 cell
monolayers. J Nutr. 2013 Dec;143(12):1872-81.
- Nunes RD, Ventura-Martins G, Moretti DM, et al. Polyphenol-Rich
Diets Exacerbate AMPK-Mediated Autophagy, Decreasing Proliferation of
Mosquito Midgut Microbiota, and Extending Vector Lifespan. PLoS Negl
Trop Dis. 2016 Oct 12;10(10):e0005034.
- Teixeira L, Ferreira A, Ashburner M. The bacterial symbiont
Wolbachia induces resistance to RNA viral infections in Drosophila
melanogaster. PLoS Biol. 2008 Dec 23;6(12):e2.
- Yang S, Long LH, Li D, et al. β-Guanidinopropionic acid extends the
lifespan of Drosophila melanogaster via an AMP-activated protein
kinase-dependent increase in autophagy. Aging Cell. 2015
Dec;14(6):1024-33.
- Ulgherait M, Rana A, Rera M, Graniel J, Walker DW. AMPK modulates
tissue and organismal aging in a non-cell-autonomous manner. Cell Rep.
2014 Sep 25;8(6):1767-80.
- Gulia-Nuss M, Robertson AE, Brown MR, Strand MR. Insulin-like
peptides and the target of rapamycin pathway coordinately regulate blood
digestion and egg maturation in the mosquito Aedes aegypti. PLoS One.
2011;6(5):e20401.
- Harrison DE, Strong R, Sharp ZD, et al. Rapamycin fed late in life
extends lifespan in genetically heterogeneous mice. Nature. 2009 Jul
16;460(7253):392-5.
- Sun X, Wheeler CT, Yolitz J, et al. A mitochondrial ATP synthase
subunit interacts with TOR signaling to modulate protein homeostasis and
lifespan in Drosophila. Cell Rep. 2014 Sep 25;8(6):1781-92.
- Roy SG, Raikhel AS. Nutritional and hormonal regulation of the TOR
effector 4E-binding protein (4E-BP) in the mosquito Aedes aegypti. FASEB
J. 2012 Mar;26(3):1334-42.
- Serbus LR, White PM, Silva JP, et al. The impact of host diet on
Wolbachia titer in Drosophila. PLoS Pathog. 2015 Mar 31;11(3):e1004777.
- Chiao YA, Kolwicz SC, Basisty N, et al. Rapamycin transiently
induces mitochondrial remodeling to reprogram energy metabolism in old
hearts. Aging (Albany NY). 2016 Feb;8(2):314-27.
- Lesniewski LA, Seals DR, Walker AE, et al. Dietary rapamycin
supplementation reverses age-related vascular dysfunction and oxidative
stress, while modulating nutrient-sensing, cell cycle, and senescence
pathways. Aging Cell. 2016 Sep 22. doi: 10.1111/acel.12524. [Epub ahead
of print].
- Wong ZS, Brownlie JC, Johnson KN. Oxidative stress correlates with
Wolbachia-mediated antiviral protection in Wolbachia-Drosophila
associations. Appl Environ Microbiol. 2015 May 1;81(9):3001-5.
- Sykiotis GP, Bohmann D. Keap1/Nrf2 signaling regulates oxidative
stress tolerance and lifespan in Drosophila. Dev Cell. 2008
Jan;14(1):76-85.
- Prochaska HJ, Fernandes CL, Pantoja RM, Chavan SJ. Inhibition of
human immunodeficiency virus type 1 long terminal repeat-driven
transcription by an in vivo metabolite of oltipraz: implications for
antiretroviral therapy. Biochem Biophys Res Commun. 1996 Apr
25;221(3):548-53.
- Kubben N, Zhang W2, Wang L, et al. Repression of the Antioxidant
NRF2 Pathway in Premature Aging. Cell. 2016 Jun 2;165(6):1361-74.
- Kim TH, Eom JS, Lee CG, Yang YM, Lee YS, Kim SG. An active
metabolite of oltipraz (M2) increases mitochondrial fuel oxidation and
inhibits lipogenesis in the liver by dually activating AMPK. Br J
Pharmacol. 2013 Apr;168(7):1647-61. doi: 10.1111/bph.12057.
- Mo C, Wang L, Zhang J, et al. The crosstalk between Nrf2 and AMPK
signal pathways is important for the anti-inflammatory effect of
berberine in LPS-stimulated macrophages and endotoxin-shocked mice.
Antioxid Redox Signal. 2014 Feb 1;20(4):574-88.
- Joo MS, Kim WD, Lee KY, Kim JH, Koo JH, Kim SG. AMPK Facilitates
Nuclear Accumulation of Nrf2 by Phosphorylating at Serine 550. Mol Cell
Biol. 2016 Jun 29;36(14):1931-42.
- Pan X, Zhou G, Wu J, et al. Wolbachia induces reactive oxygen
species (ROS)-dependent activation of the Toll pathway to control dengue
virus in the mosquito Aedes aegypti. Proc Natl Acad Sci U S A. 2012 Jan
3;109(1):E23-31.
- Nunes RD, Ventura-Martins G, Moretti DM, et al. Polyphenol-Rich
Diets Exacerbate AMPK-Mediated Autophagy, Decreasing Proliferation of
Mosquito Midgut Microbiota, and Extending Vector Lifespan. PLoS Negl
Trop Dis. 2016 Oct 12;10(10):e0005034.
- Li GX, Chen YK, Hou Z, et al. Pro-oxidative activities and
dose-response relationship of (-)-epigallocatechin-3-gallate in the
inhibition of lung cancer cell growth: a comparative study in vivo and
in vitro. Carcinogenesis. 2010 May;31(5):902-10.
- Chang YF, Chi CW, Wang JJ. Reactive oxygen species production is
involved in quercetin-induced apoptosis in human hepatoma cells. Nutr
Cancer. 2006;55(2):201-9.
- Miki H, Uehara N, Kimura A, et al. Resveratrol induces apoptosis
via ROS-triggered autophagy in human colon cancer cells. Int J Oncol.
2012 Apr;40(4):1020-8.
- Ullah MF, Ahmad A, Zubair H, et al. Soy isoflavone genistein
induces cell death in breast cancer cells through mobilization of
endogenous copper ions and generation of reactive oxygen species. Mol
Nutr Food Res. 2011 Apr;55(4):553-9.
- Matsuyama S, Moriuchi M1, Suico MA, et al. Mild electrical
stimulation increases stress resistance and suppresses fat accumulation
via activation of LKB1-AMPK signaling pathway in C. elegans. PLoS One.
2014 Dec 9;9(12):e114690.
- Dutra HL, Rocha MN, Dias FB, Mansur SB, Caragata EP, Moreira LA.
Wolbachia Blocks Currently Circulating Zika Virus Isolates in Brazilian
Aedes aegypti Mosquitoes. Cell Host Microbe. 2016 Jun 8;19(6):771-4.
- Zandi K, Teoh BT, Sam SS, Wong PF, Mustafa MR, Abubakar S.
Antiviral activity of four types of bioflavonoid against dengue virus
type-2. Virol J. 2011 Dec 28;8:560.
- Han YS, Penthala NR2, Oliveira M, et al. Identification of
resveratrol analogs as potent anti-dengue agents using a cell-based
assay. J Med Virol. 2016 Aug 10. doi: 10.1002/jmv.24660. [Epub ahead of
print].
- Carneiro BM, Batista MN, Braga AC, Nogueira ML, Rahal P. The green
tea molecule EGCG inhibits Zika virus entry. Virology. 2016
Sep;496:215-8.
- Padilla-S L, Rodríguez A, Gonzales MM, Gallego-G JC, Castaño-O JC.
Inhibitory effects of curcumin on dengue virus type 2-infected cells in
vitro. Arch Virol. 2014 Mar;159(3):573-9.
- Sharma N, Mishra KP, Ganju L. Salidroside exhibits anti-dengue
virus activity by upregulating host innate immune factors. Arch Virol.
2016 Dec;161(12):3331-3344.
- Chang X, Zhang K, Zhou R, et al. Cardioprotective effects of
salidroside on myocardial ischemia-reperfusion injury in coronary artery
occlusion-induced rats and Langendorff-perfused rat hearts. Int J
Cardiol. 2016 Jul 15;215:532-44.
- Ejaz A, Wu D, Kwan P, Meydani M. Curcumin inhibits adipogenesis in
3T3-L1 adipocytes and angiogenesis and obesity in C57/BL mice.
- Pellegrini C, Columbaro M, Capanni C, et al. All-trans retinoic
acid and rapamycin normalize Hutchinson Gilford progeria fibroblast
phenotype. Oncotarget. 2015 Oct 6;6(30):29914-28.
- Fernandez-Garcia MD, Meertens L, Bonazzi M, Cossart P,
Arenzana-Seisdedos F, Amara A. Appraising the roles of CBLL1 and the
ubiquitin/proteasome system for flavivirus entry and replication. J
Virol. 2011 Mar;85(6):2980-9.
- Jiang S, Park DW, Gao Y, et al. Participation of
proteasome-ubiquitin protein degradation in autophagy and the activation
of AMP-activated protein kinase. Cell Signal. 2015 Jun;27(6):1186-97.
- Kreienkamp R, Croke M, Neumann MA, et al. Vitamin D receptor
signaling improves Hutchinson-Gilford progeria syndrome cellular
phenotypes. Oncotarget. 2016 Apr 27. doi: 10.18632/oncotarget.9065.
- Puerta-Guardo H, Medina F, De la Cruz Hernández SI, Rosales VH,
Ludert JE, del Angel RM. The 1α,25-dihydroxy-vitamin D3 reduces dengue
virus infection in human myelomonocyte (U937) and hepatic (Huh-7) cell
lines and cytokine production in the infected monocytes. Antiviral Res.
2012 Apr;94(1):57-61.
- Swami S, Krishnan AV, Williams J, et al.. Vitamin D mitigates the
adverse effects of obesity on breast cancer in mice. Endocr Relat
Cancer. 2016 Apr;23(4):251-64.
- Piette J, Legrand-Poels S. HIV-1 reactivation after an oxidative
stress mediated by different reactive oxygen species. Chem Biol
Interact. 1994 Jun;91(2-3):79-89.
- Tamás P, Hawley SA, Clarke RG, et al. Regulation of the energy
sensor AMP-activated protein kinase by antigen receptor and Ca2+ in T
lymphocytes. J Exp Med. 2006 Jul 10;203(7):1665-70.
- LaRosa C, Downs SM. Stress stimulates AMP-activated protein kinase
and meiotic resumption in mouse oocytes. Biol Reprod. 2006
Mar;74(3):585-92.
- Patrat C, Serres C, Jouannet P. The acrosome reaction in human spermatozoa. Biol Cell. 2000 Jul;92(3-4):255-66.
- Calle-Guisado V, de Llera AH, Martin-Hidalgo D, et al.
AMP-activated kinase in human spermatozoa: identification, intracellular
localization, and key function in the regulation of sperm motility.
Asian J Androl. 2016 Sep 27. doi: 10.4103/1008-682X.185848. [Epub ahead
of print].
- de Lamirande E, Tsai C, Harakat A, Gagnon C. Involvement of
reactive oxygen species in human sperm arcosome reaction induced by
A23187, lysophosphatidylcholine, and biological fluid ultrafiltrates. J
Androl. 1998 Sep-Oct;19(5):585-94.
- Blomberg Jensen M, Bjerrum PJ, Jessen TE, et al. Vitamin D is
positively associated with sperm motility and increases intracellular
calcium in human spermatozoa. Hum Reprod. 2011 Jun;26(6):1307-17.
- Finley J. Oocyte activation and latent HIV-1 reactivation: AMPK as a
common mechanism of action linking the beginnings of life and the
potential eradication of HIV-1. Med Hypotheses. 2016 Aug;93:34-47.
- Soto-Acosta R, Bautista-Carbajal P, Cervantes-Salazar M,
Angel-Ambrocio AH, Del Angel RM. DENV up-regulates the HMG-CoA reductase
activity through the impairment of AMPK phosphorylation: A potential
antiviral target. PLoS Pathog. 2017 Apr 6;13(4):e1006257.
- Park SK, Shin OS. Metformin Alleviates Aging Cellular Phenotypes in
Hutchinson-Gilford Progeria Syndrome Dermal Fibroblasts. Exp Dermatol.
2017 Feb 13. doi: 10.1111/exd.13323. [Epub ahead of print].
- Egesipe, Blondel, Cicero, et al. Metformin decreases progerin
expression and alleviates pathological defects of Hutchinson–Gilford
progeria syndrome cells. npj Aging and Mechanisms of Disease 2, Article number: 16026 (2016); http://www.nature.com/articles/npjamd201626?WT.feed_name=subjects_drug-discovery
- Finley J. Alteration of splice site selection in the LMNA gene and
inhibition of progerin production via AMPK activation. Med Hypotheses.
2014 Nov;83(5):580-7.
- Deemeh MR, Tavalaee M, Nasr-Esfahani MH. Health of children born
through artificial oocyte activation: a pilot study. Reprod Sci. 2015
Mar;22(3):322-8.
- Finley J. Reactivation of latently infected HIV-1 viral reservoirs
and correction of aberrant alternative splicing in the LMNA gene via
AMPK activation: Common mechanism of action linking HIV-1 latency and
Hutchinson-Gilford progeria syndrome. Med Hypotheses. 2015
Sep;85(3):320-32.